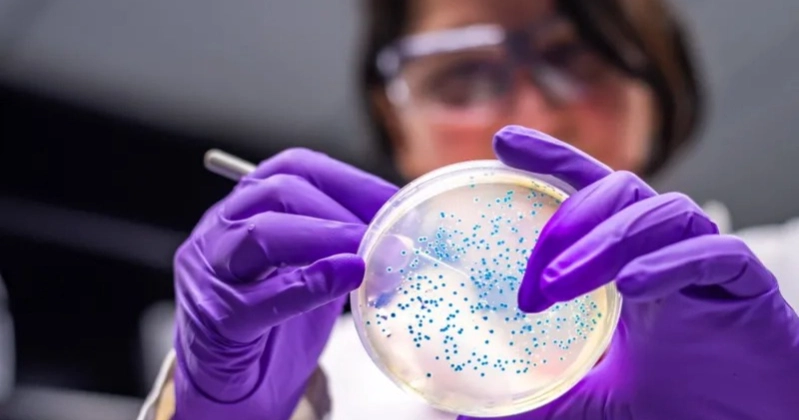
Earlier this year, scientists reported a breakthrough in biotechnology. A common bacterium was genetically engineered to convert plastic waste into the widely used painkiller paracetamol.
The bacterium, Escherichia coli (E. coli), was modified by Stephen Wallace, professor of chemical biotechnology at the University of Edinburgh, to digest a plastic-derived molecule and transform it into the drug.
While E. coli is often associated with food poisoning, non-pathogenic strains are widely used in laboratories as the field’s main “workhorse.”
Prof Wallace has previously engineered E. coli to turn plastic waste into vanilla flavoring and sewer “fatberg” waste into perfume. “If you want to prove something is possible with biology, E. coli is a natural first stage,” he said.
Beyond laboratories, vats of engineered E. coli serve as living factories, producing vital substances such as insulin, which is essential for diabetes treatment, as well as chemicals for fuels and solvents.
Why E. coli dominates biotechnology
According to Princeton University professor Thomas Silhavy, E. coli’s dominance began with its use as a model organism to understand basic biology. First isolated in 1885 by German pediatrician Theodor Escherich, its rapid growth and ease of handling made it a natural choice for research.
In the 1940s, studies using a harmless E. coli strain revealed that bacteria could exchange genes through a process akin to “bacterial sex,” reshaping the understanding of microbial genetics. Since then, E. coli has been central to major discoveries, from deciphering the genetic code to becoming the first organism genetically engineered with foreign DNA in the 1970s.
The bacterium was also behind a breakthrough in 1978, when synthetic human insulin was first produced using E. coli, eliminating the reliance on animal-derived insulin that sometimes caused allergic reactions. Nearly two decades later, in 1997, E. coli was among the first organisms to have its entire genome sequenced.
E. coli’s strengths
Adam Feist, professor at the University of California, San Diego, highlights the bacterium’s speed, reliability, and versatility. It thrives on a wide variety of substrates, can be frozen and revived easily, and efficiently hosts foreign DNA. “The more I work with other microorganisms, the more I appreciate just how robust E. coli is,” he said.
Cynthia Collins of Ginkgo Bioworks notes that while more organisms are now available for industrial use, E. coli remains cost-effective and adaptable. Even when producing potentially toxic substances, scientists can often engineer tolerance into the bacterium.
Calls for alternatives
Some experts warn that E. coli’s dominance may limit exploration of other promising microbes. Paul Jensen, a microbiologist at the University of Michigan, argues that undiscovered bacteria in landfills or natural environments might naturally perform valuable processes, such as breaking down plastics or even producing new materials like cement or rubber.
One emerging alternative is Vibrio natriegens (V. nat), first isolated in the 1960s but largely overlooked until recently. With a growth rate twice as fast as E. coli and superior ability to absorb foreign DNA, V. nat could offer significant industrial advantages. Cornell University’s Buz Barstow, who is developing tools to engineer it, compares the leap from E. coli to V. nat as “going from a horse to a car.”
His team has launched a company, Forage Evolution, to advance its applications, with ambitions ranging from producing jet fuel from carbon dioxide to extracting rare earth metals. “Simply put, E. coli won’t get us to any of these visions. V. natriegens might,” Dr Barstow said.
Still, experts like Prof Feist caution that V. nat is not yet ready to replace E. coli at scale, as the essential genetic tools and industrial track record are still lacking. “E. coli is a tough thing to replace,” he said.



-20260228080513.webp)
-20260224065127.webp)
-20260222063838.webp)







-20260302065048.webp)













-20260224075258.webp)



-20260225072312.webp)
