Bangladesh Medical Research Council (BMRC) has approved local pharmaceutical company Globe Biotech to launch human trials of Bangavax, the first Covid-19 vaccine developed in Bangladesh.
On Tuesday (November 23), Dr Md Mohiuddin, senior manager (Quality and Regulation) of Globe Biotech confirmed the matter to the media.
To begin the trial, Globe Biotech will apply to the Directorate General of Drug Administration (DGDA), said Dr. Md Mohiuddin.
Globe Biotech will now be able to conduct Phase I of the trial maintaining the conditions set by the BMRC, added the official.
If Phase-II and Phase-III trials show the shot is effective and safe enough, it will get clearance for widespread use
Bangavax is a single dose Covid-19 vaccine developed in Bangladesh by local pharmaceutical Globe Biotech, and is also the first experimental vaccine to be approved for human trial in the country.


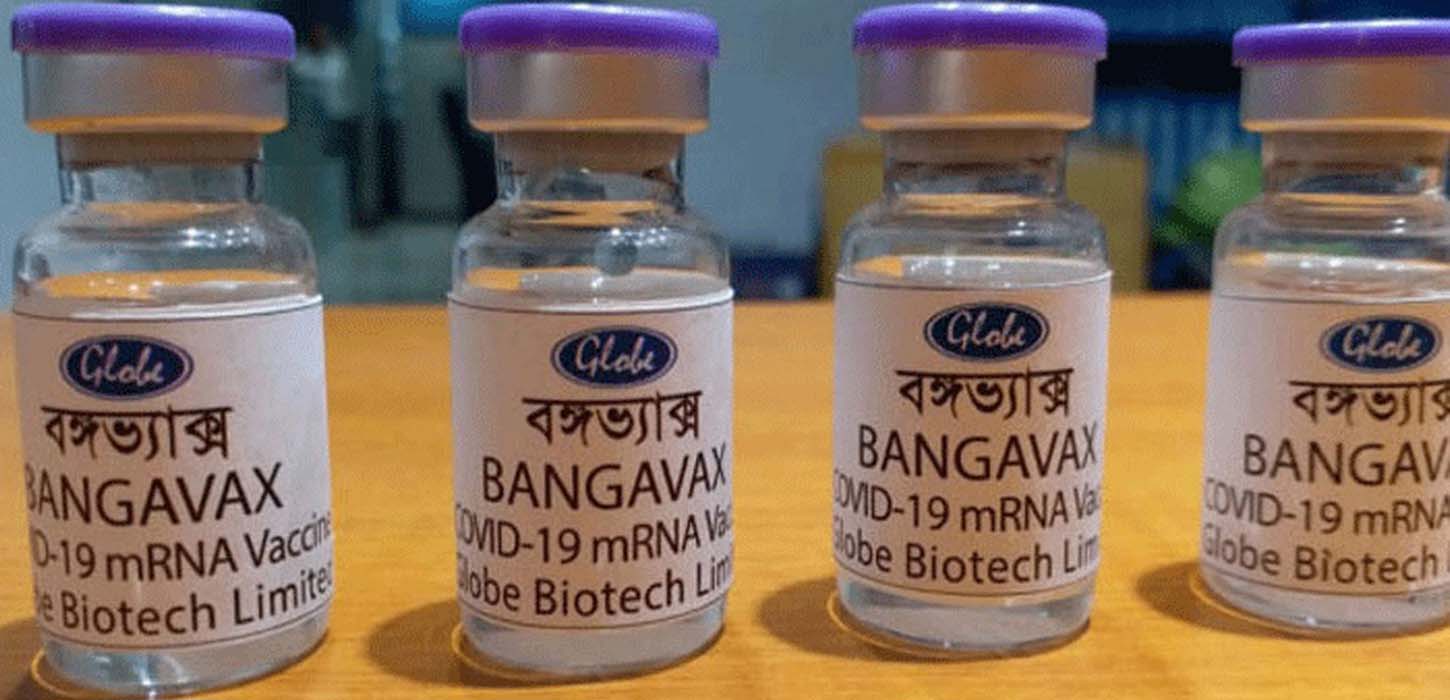










-20260226080139.webp)




-20260225072312.webp)






-20260219054530.webp)
-20260224075258.webp)





-20260221022827.webp)