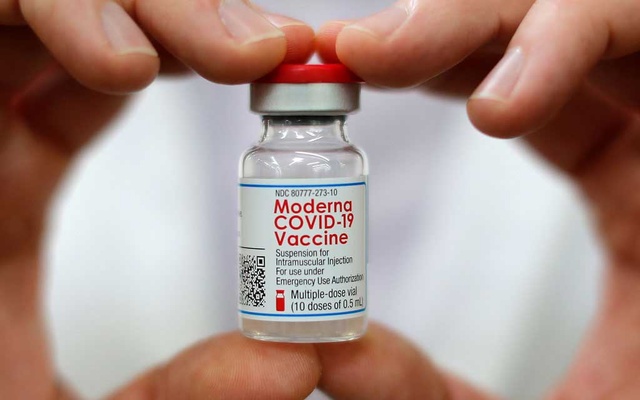
The drug company Moderna has begun a study that will test its COVID vaccine in children younger than 12, including babies as young as 6 months, the company said Tuesday.
The study is expected to enrol 6,750 healthy children in the United States and Canada. Moderna declined to say how many had already signed up or received the first shots, according to a spokeswoman, Colleen Hussey.
“There’s a huge demand to find out about vaccinating kids and what it does,” said Dr David Wohl, medical director of the vaccine clinic at the University of North Carolina, who is not involved in the study.
In a separate study, Moderna is testing its vaccine in 3,000 children ages 12-17 and may have results for that age group by summer. The vaccine would then have to be authorised for use in children, so it would not be immediately available.
Many parents want protection for their children, and vaccinating children should help to produce herd immunity considered crucial to stopping the pandemic. The American Academy of Pediatrics has called for the expansion of vaccine trials to include children.
Vaccine side effects like fever, sore arms, fatigue and achy joints and muscles can be more intense in children than in adults, and doctors say it is important for parents to know what to expect after their children are inoculated.
Each child in Moderna’s study will receive two shots, 28 days apart. The study will have two parts. In the first, children ages 2 years to younger than 12 may receive two doses of 50 or 100 micrograms each. Those younger than 2 may receive two shots of 25, 50 or 100 micrograms. (One adult dose is 100 micrograms.)
In each group, the first children inoculated will receive the lowest doses and will be monitored for reactions before later participants are given higher doses.
Then, researchers will perform an interim analysis to determine which dose is safest and most likely to be protective for each age group.
Children in part two of the study will receive the doses selected by the analysis — or placebo shots consisting of saltwater.
Moderna developed its vaccine in collaboration with the National Institute of Allergy and Infectious Diseases. The company and the institute are also working together on the study, along with the federal Biomedical Advanced Research and Development Authority.
The children will be followed for a year, to look for side effects and measure antibody levels that will help researchers determine whether the vaccine appears to provide protection. The antibody levels will be the main indicator, but the researchers will also look for coronavirus infections, with or without symptoms.
Wohl said the study appeared well designed and likely to be efficient, but he questioned why the children were to be followed for only one year, when adults in Moderna’s study are followed for two years. He also said he was somewhat surprised to see the vaccine being tested in children so young this soon.
Should we learn first what happens in the older kids before we go to the really young kids?” Wohl asked.
Most young children do not become very ill from COVID, he said, although some develop a severe inflammatory syndrome that can be life threatening.
Johnson & Johnson has also said it would test its coronavirus vaccine in babies and young children after testing it first in older children.
Pfizer and BioNTech are testing their vaccine in children ages 12-15 and have said the plan is to move to younger groups; the product is already authorised for use in those 16 and older in the United States.
Last month, AstraZeneca began testing its vaccine in Britain in children 6 years and older.
© 2021 The New York Times Company



-20260305071113.webp)
-20260304091720.webp)



-20260303080739.webp)




















-20260228064648.jpg)
